R&D Pipeline and
Development Status
- Project Candidate
- Target Disease
- Discovery
- Preclinical
- IND
- Phase 1
- Phase 2
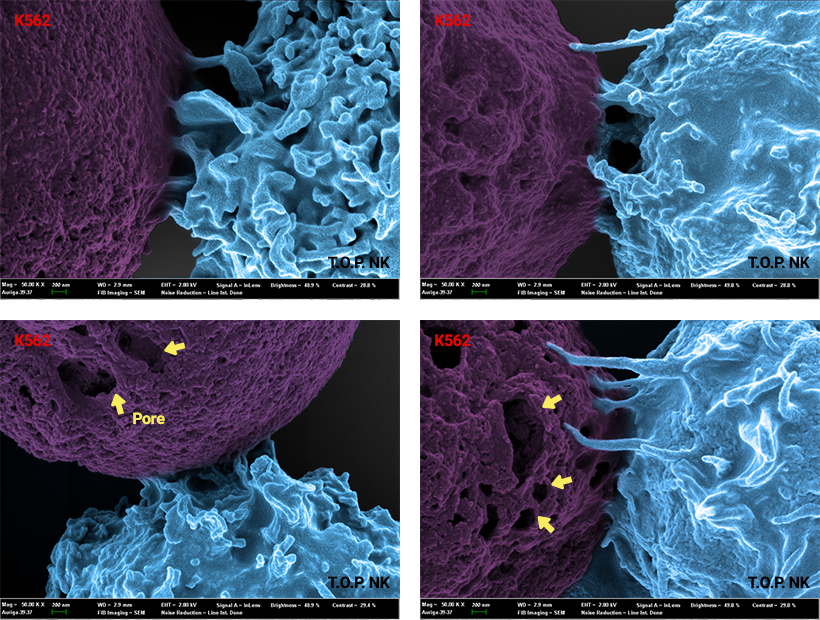
GIC-102
(T.O.P. NK®)
Solid / Hematologic cancers
- Description : NK cell therapy activated and amplified using ONLY the ancillary proteins. Without genetic modification or viral transduction, CD16 expression and cytotoxic granule contents are HIGHLY ELEVATED in T.O.P. NK cells.
- Technology : NKPURE Expander® Platform
- Partnership Proposal : T.O.P. NK® Platform (T.O.P. NK® is the cell-based platform. We can develop the platform to various cell therapies like CAR-NK, Nano NK)
GIC-101
(Nano NK®)
Solid cancers
- Description : Allogeneic NK cell reinforced with chemo-loaded nanoparticles
- Technology : NKPURE Expander® Platform, T.O.P. NK®
GIC-302
(Drone Treg®)
Inflammatory Bowel Disease
- Description : Autologous regulatory T cell highly expressing organ specific homing receptors for the treatment of inflammatory disease
- Technology : TregPure ExpanderTM Platform
- Partnership Proposal : Drone Treg® Platform (Drone Treg® is the cell-based platform. We can develop the platform to various cell therapies like CAR-Treg)
CAR NK
Solid/ Hematologic cancer
- Description :Allogeneic NK cell therapies with Chimeric Antigen Receptors(CAR)
- Technology : NKPURE Expander® Platform
- Partners : HK inno.N, Cartexell, T.O.P. NK®

 ENG
ENG