Platform
Immune CellPure Expander® Platform
-

High Safety
-

High Efficacy
-

High Scalability
-

High Versatility
Customizable, Protein-based Cell Programing Platform
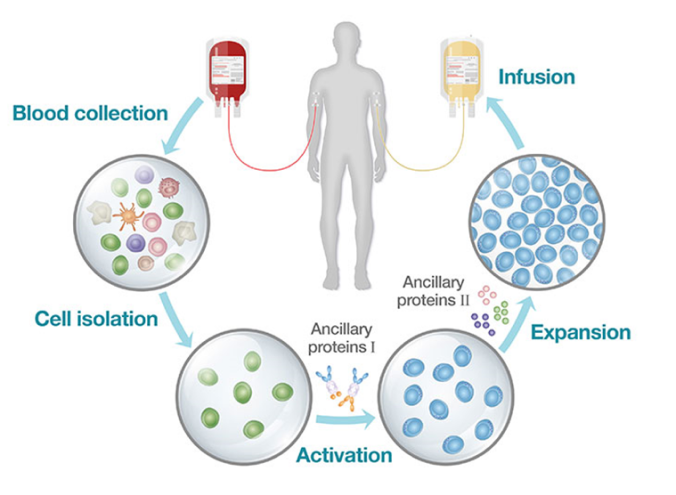
Encompassing technologies and capabilities across the full continuum of NK and
T cell therapy process development & manufacturing
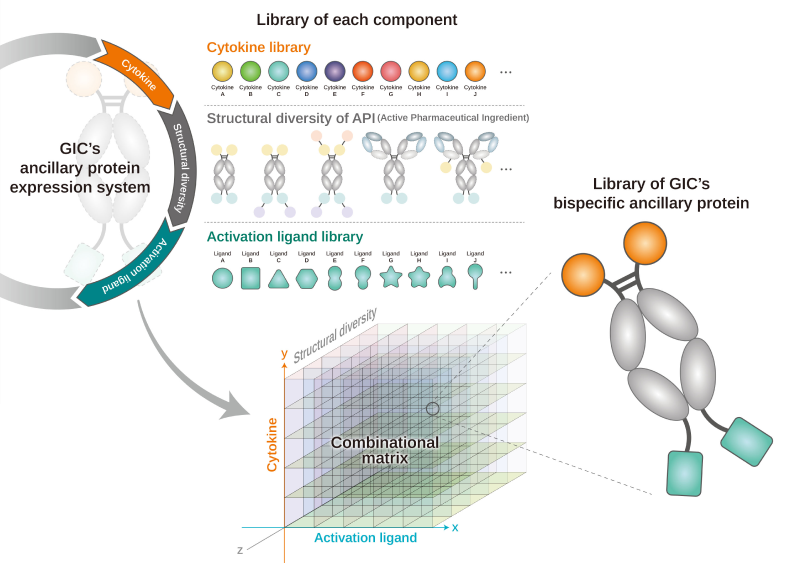
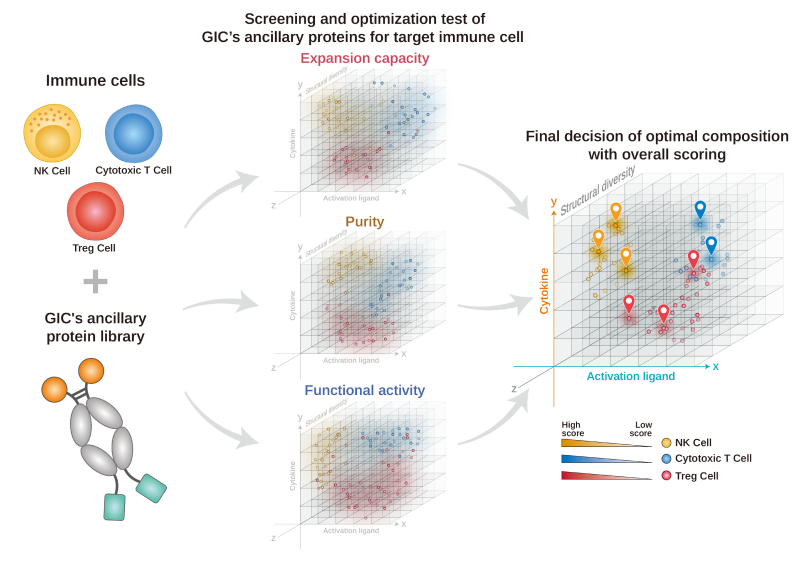
-
Ancillary protein library for
immune cell 
-
Database of candidate screening
and feasibility test 
-
Optimization of immune
cell-specific manufacturing
Technology
T.O.P. NK®
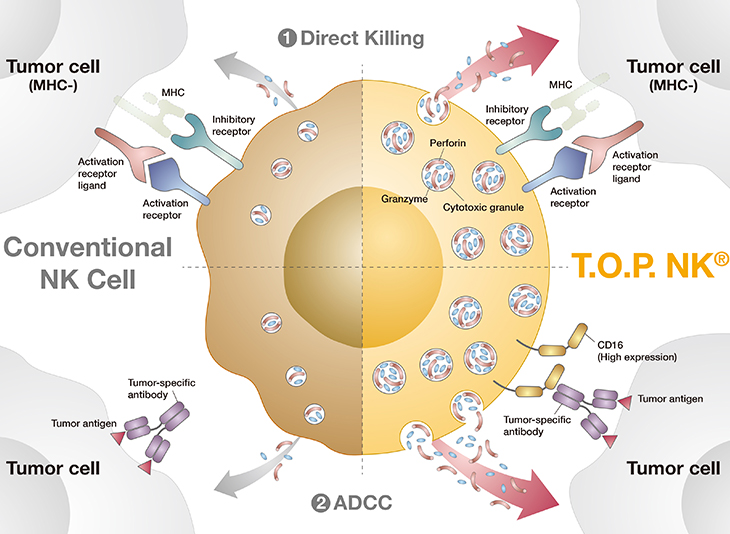
Key features of T.O.P. NK®
- Tumor-targeting Optimally Primed (T.O.P.) NK Cell.
- T.O.P. NK cells are robustly activated and amplified using ONLY the ancillary proteins in the NKPURE Expander® platform.
- T.O.P. NK cells are highly pure (>99%) and safe to use as NO feeder cells are used during the cultivation process.
- Even with NO genetic modification or viral transduction, CD16 (FcγRIII) expression and cytotoxic granule contents are HIGHLY ELEVATED in T.O.P NK cells.
- High CD16 expression enables T.O.P. NK cells to target and kill tumor cells via antibody-dependent cell cytotoxicity (ADCC).
- Robust cytotoxic granule contents elevate T.O.P. NK cells’ killing competency.
Nano NK®
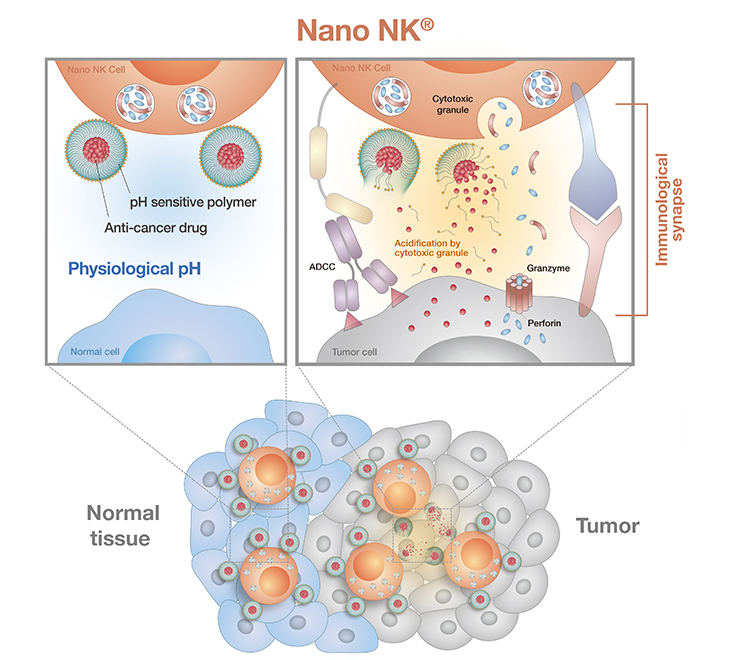
Key features of Nano NK®
- Nanoparticle-armed (Nano) NK Cells are NK cells carrying pH-sensitive nanoparticles loaded with a chemotherapeutic drug.
- Tumor-specific homing and targeting by the NK cells grants major advantages over conventional chemotherapy, which causes systemic adverse effect to the recipients.
- Nano NK cells carry out tumor-targeted drug delivery, which significantly reduce the toxicity of released chemotherapeutic agent.
- Upon engaging a tumor cell, Nano NK cell releases its cytotoxic granules, causing the immunological synapse between engaging NK cell and target tumor cell to turn acidic.
- Acidification of the surroundings prompts nanoparticle disassembly and release of chemotherapeutic drug directly onto the tumor cell, resulting in tumor cell killing.
- This technology could also be adapted to reinforce cytotoxic T lymphocytes with chemo-loaded nanoparticles.
Drone Treg®
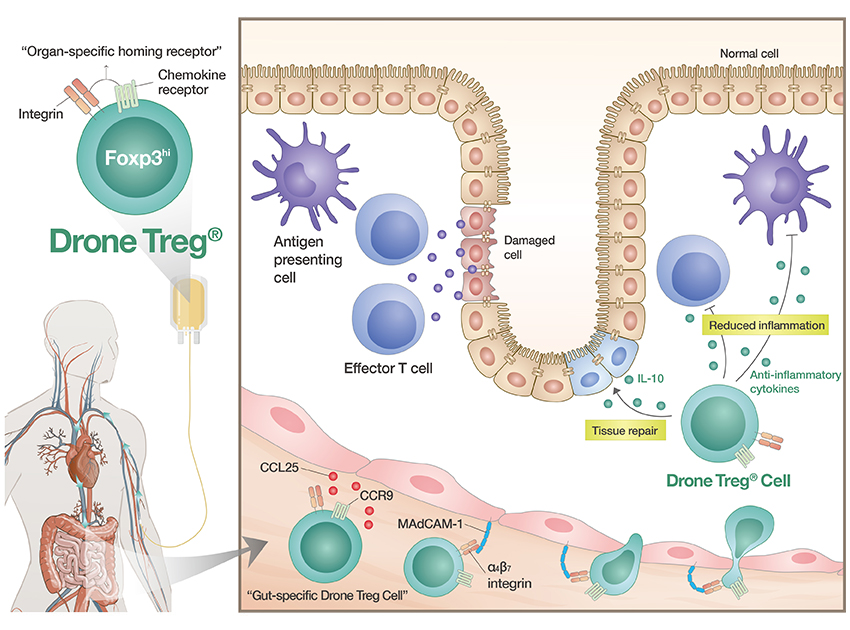
Key features of Drone Treg®
- Regulatory T cells play important role in maintaining tolerance and immune cell homeostasis.
- Tregs are crucial in controlling chronic inflammatory diseases as they can suppress inflammatory immune responses through secretion of inhibitory cytokines.
- Target organ-specific regulatory T cell therapy platform which induces controlled expression of organ-specific homing receptors from the Foxp3hi regulatory T cell isolated from patient’s blood.
- Drone Treg® expressing gut-specific homing markers, CCR9 and α4β7 integrin, induce migration to lesion of patient’s gut after infusion.
- In intestinal autoimmune diseases, APCs and effector T cells elicit and propagate aberrant inflammatory responses towards innocuous antigens, such as food particles or commensal microbiomes, which leads to tissues damage in the gut.
- Drone Treg® in the gut can effectively control the inflammatory APCs and effector T cells by releasing anti-inflammatory cytokines and induce repair of damaged tissues.

 ENG
ENG